How often do we hear that chemistry surrounds and involves us and how unconvinced are we? Yet, one only has to look at our nail polish-embellished nails to be in fact in front of a small chemistry laboratory.

Nail polish is a special synthetic lacquer that is used to colour fingernails and toenails.
The custom of painting one’s nails dates back to the third millennium B.C. when the ancient Chinese applied a mixture of plant substances such as flower petals and gum arabic, animal substances such as beeswax and minerals as colouring agents to the nails as a sign of distinction of the rich class. The ancient Egyptians of the upper classes coloured their nails with henna, which gave them a typical red-brown colour. The first nail polishes with an exclusively aesthetic purpose, and not as a sign of social class distinction, only appeared from the 9th century onwards.
Commonly, an nail polish is made up of a solution of nitrocellulose, a thermoplastic polymer also known as cellulose nitrate or fulmicotone, which forms a film that imparts the typical smooth enamel surface, dissolved in ethyl acetate or butyl acetate, two esters of acetic acid (do exercise 43) . Fundamental components such as inorganic pigments are then added to this solution to impart the different colours to the glazes: chromium oxide is the compound for green colours, iron oxide for reds and shades of orange and ferric ferrocyanide for blue variations. Organic pigments are also mixed, a wide range of colours produced from compounds very similar to food colourants.
To achieve a pearly effect, pulverised titanium dioxide, mica or even tiny bits of glitter and thickeners are mixed with the enamel to prevent the different components from separating, making the enamel easier to apply. The most commonly used thickener is stearalkonium hectorite.
Benzophenone-1, a polishing agent that contains ultraviolet filters to prevent discolouration of the nail polish when the nails are exposed to sunlight, is also often added. In fact, this additive absorbs and filters ultraviolet light and prevents the pigment that colours nail polish from bleaching.
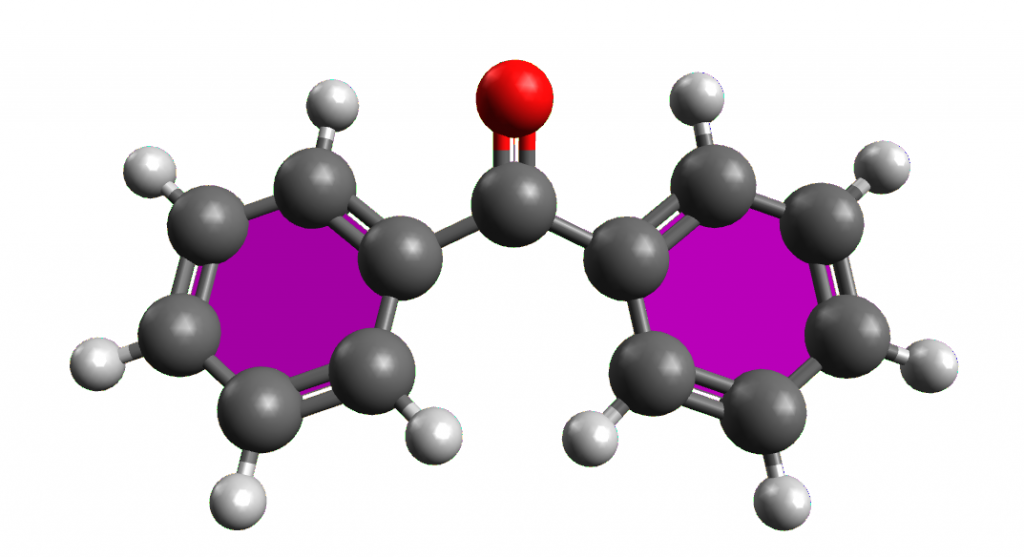
When nail polish is applied to the nail, the solvent evaporates quickly: the amount and the solvent used by the manufacturer determine the thickness of the nail polish applied and the time for it to dry. Cosmetic research has led to the realisation that products such as toluene, xylene and formaldehyde, once used as solvents in nail polish, are toxic and have therefore been replaced by ethyl and butyl acetates. To prevent the nail polish from chipping, plasticisers are then added, in particular camphor, which have the function of attaching themselves to the polymer chains so as to distance them to make the nail and the polish flexible after drying.
In recent years, the application of semi-permanent nail polish, i.e. resins that make the nail polish particularly resistant and thus long-lasting, has developed greatly. Semi-permanent nail polish is very similar to a classic nail polish, but it must be treated, once applied on the nails, with a LED or UV lamp that favours the polymerisation of the resins, i.e. the drying and hardening of the nail polish, which thus extends its duration by up to three weeks without fading or chipping. The resins used are based on formaldehyde and tosylamide, a synthetic polymer of formaldehyde. Formaldehyde is present in semi-permanent nail polishes because it acts as a hardener and makes the nail polish more resistant. However, formaldehyde is reactive, toxic and has carcinogenic activity. Studies show that semi-permanent nail polish is more toxic and harmful than normal nail polish as it contains toxic substances such as toluene, phthalates, and of course formaldehyde. In addition, exposure to UV rays to help hardening increases the risk of skin cancer, just as it does when tanning without a protective cream. Lastly, the removal of semi-permanent nail polish is done with solvents and the use of files that often remove the horny layer of the nail that is no longer recovered.
It is therefore important to carefully read the INCI (International Nomenclature of Cosmetics Ingredients) of nail polishes and check for the hazardous substances mentioned. In fact, some cosmetics manufacturers have developed nail polishes, even semi-permanent ones, which are free of toxic substances that, moreover, can be removed without filing the nail, thus avoiding damaging it.
ACTIVITY: Learn more about the composition of nail polishes. Create a list specifying the toxicity of each component.