A sprain while running, a blow in a football match, the extraction of a tooth – there are many occasions when pain and swelling are relieved by applying ice to the painful area. Cold, in fact, relieves and decongests the affected area due to the vasoconstriction effect on the local blood supply and the decrease in blood flow, thus helping to limit and reduce oedema. Cold also has an analgesic and myorelaxant effect because it reduces the speed of the pain stimulus at the peripheral nerve level.
Today, the traditional bag full of ice cubes is being replaced by bags of instant ice, which is just as effective on bruises, but more convenient and practical as it does not require a freezer to provide an immediate source of cold.
Instant ice bags are generally made of plastic, polyethylene or PVC, and are operated by pressing firmly on a designated spot to break an internal bubble. Subsequently, shaking them achieves instant cooling.
But what does chemistry have to do with it?
Inside the bag are a few grams of ammonium nitrate (NH4NO3) and a bag containing water. The proportion of ammonium nitrate to water is 1.5 g of salt per gram of water. When you press down hard on the bag, the bag of water inside is ruptured, and the contact between the water and the ammonium nitrate triggers a highly endothermic reaction, which results in considerable heat absorption from the external environment, and the bag, then applied to the sore area, is kept at a temperature of between -5 °C and 0 °C for about 30 minutes.
The solubilisation reaction of the ammonium nitrate that takes place inside the pouch is:
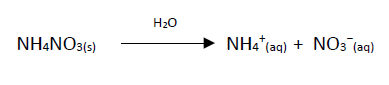
For every mole of ammonium nitrate that reacts, there is an energy absorption of 23kJ.
Another way to achieve instantaneous refrigeration comes from spray ice cans, which are based not on a reaction but on the use of a non-toxic and non-flammable substance, isobutane, which, when sprayed on the bruised part, produces the same effect as ice due to a high latent heat of evaporation and a low boiling temperature.
The propellant is a mixture of propane-butane gas, while the addition of menthol fragrance makes the smell more pleasant. Spray ice should not, however, be kept on the skin of the injured part for a long time because it can cause burns. For the same reason, it is important not to spray too directly, but to hold the can at a distance of at least 30 cm from the area to be treated and at an angle of about 30°. Finally, it should be remembered that spray ice is soothing and not curative.
ACTIVITY: research and write a short text about the chemical properties of ammonium nitrate, how it is obtained industrially and its applications in different fields.