Rare earths are the 17 elements of the periodic table that correspond to the lanthanides plus scandium, yttrium and lanthanum, which belong to group 3B.
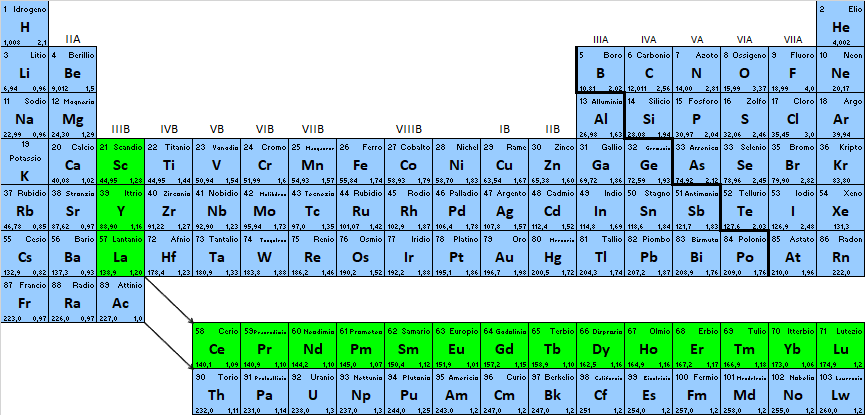
Rare earths is the name given to minerals that were first mined in a quarry in Sweden at the end of the 18th century, the oxides of which were indeed infrequent. But they are also found associated with other elements in the form of carbonates, silicates and phosphates. Rare earths are classified as light rare earths (abbreviated to LREE, Low Rare Earth Elements) to which the elements from lanthanum to promethium belong, medium rare earths (MREE) ranging from samarium to holmium, and heavy rare earths (HREE) from erbium to lutetium.
In spite of their name, rare earths are widespread in the earth’s crust. For example, cerium is as abundant as copper and thulium and lutetium are 200 times more abundant than gold. However, there are no deposits of rare earths alone, but of minerals that contain them as oxides.
The electronic configuration of the lanthanides involves the full or partial filling of the 4f orbitals, except for the elements belonging to group 3B, which fill the d orbitals. Since the f orbitals have little effect on the chemical properties of an element, all lanthanides exhibit basically the same behaviour and characteristics. For the entire lanthanide series, the characteristic oxidation state is i +3, which also gives rise to highly coloured solutions.

Lanthanides do not receive much attention in the teaching of chemistry up to university level, yet their applications are wide, complex and diverse.
Suffice it to say that parts of most of the electronic devices we use on a daily basis are based on precisely those elements that belong to the rare earths. For example, mobile phones contain tiny amounts of lanthanum, neodymium, terbium, gadolin and other elements that play a key role in colour display, sound reproduction or vibration systems. Despite being only 1 per cent by weight, as many as 16 out of 17 rare earths are used in an iPhone and are crucial to its operation. Due to their electrochemical, magnetic and optical properties, rare earths are also present and essential in computer hard disks, touchscreens, fibre optics and lasers, many medical devices, electric car batteries, aerospace and the military.
To understand their importance in our lives, one only has to think that if they were to be completely depleted, there would be a major setback in the performance standards of electronic devices.
In addition to the aforementioned uses, the elements europium and terbium are used in banknotes to prevent them from being counterfeited, neodymium alloys to make the permanent magnets in headphones and loudspeakers, europium, cesium, terbium and gadolinium phosphor compounds in TV screens for their luminescence, also in the form of compounds are the colouring agents in ceramic glass, and finally samarium and cobalt alloys make up the magnets found in electric guitar pick-ups.
Due to the sudden rise in the use of rare earths in various applications, they are gradually being depleted, as is the case in the United States where they are almost completely exhausted. To date, China is the world’s main source of these elements with 55% of the world’s extraction capacity and 85% of their refining, as well as 37% of the world’s reserves. Other reserves, albeit far behind China in percentage terms, are the United States (12.3%), Myanmar (10.5%) and Australia (10%).
Rare earth mining and refining present several environmental challenges. In fact, the processes for separating the individual elements involve the use of hydrochloric acid and nitric acid as solvents, processes that have a very strong environmental impact, resulting in soil and groundwater pollution. Naturally, they are among the non-renewable resources and, at present, only 1% can be recycled with difficulty.
Controlling rare earths is crucial for the future of green economies. Since Europe is dependent in this sector on the great powers China and the United States, which are trying to grab the deposits that are becoming accessible due to the thawing of the permafrost, it has set up the European Raw Materials Alliance ‘to build resilience and strategic autonomy on rare earths’. A manoeuvre to act on sustainability and global social impact.
ACTIVITY: Identify all the Agenda 2030 goals related to the topics discussed in the article.